
BENESOMNOTM has been approved as a Health Functional Food (HFF) ingredient by the Ministry of Food and Drug Safety (MFDS) of Korea

BENESOMNO™ is a nutraceutical and dietary ingredient for sleep developed by Nutra-it Inc., a company with world-class expertise in sleep science, to compete in the global market.
The name BENESOMNO™ combines the Latin words "BENE" (GOOD) and "SOMNO" (SLEEP), reflecting Nutra-it Inc.’s vision to fostering a healthier world through better sleep.


- Nutra-it's research team possesses world-class expertise in sleep-promoting ingredients and has successfully developed Ecklonia cava extract and rice bran extract, both approved as sleep-promoting nutraceuticals by the Korean FDA.
- Focusing on the development of globally recognized sleep-promoting ingredients, Nutra-it successfully developed BENESOMNOTM, an innovative sleep-promoting ingredient derived from lime peel.
- BENESOMNOTM is produced using a patented process that isolates only the active compounds with scientifically proven sleep-promoting effects from the peels of Mexican lime (Citrus aurantifolia).
- Nutra-it applies a systematic and scientific quality management system throughout the entire production process, from sourcing lime peels to the final production of BENESOMNOTM.
- BENESOMNOTM has been patented in Korea and has patent applications filed in the United States, Europe, and China.
| Efficacy | Excellent |
| Mechanism of action | GABA agonist (GABAA receptor) |
| Adverse effects | No |
| Dosage | 300 mg/day (Lime extract 150 + Dextrin 150) |
| Raw material | Mexican Key lime peel (Non-GMO) |
| Marker compound | Vicenin-2 (flavonoid) |
| Available dosage forms | Gummy, Granule, Tablet, Liquid etc. |
- This study aimed to evaluate the effects of SLPS in adults experiencing sleep disturbances. The randomized, double-blind, placebo-controlled clinical trial involved 80 subjects who received either SLPS (300 mg/day) or placebo for a 2-week period.
- SLPS significantly improved polysomnographic outcomes, including a reduction in sleep latency, wake after sleep onset, and total wake time, and enhancement of sleep efficiency, total sleep time, and stage 2 sleep. Daytime sleepiness, assessed via the Epworth Sleepiness Scale, was also decreased by SLPS.
- No serious adverse effects or side effects were reported among participants in the SLPS group during the intervention period. Our findings support SLPS as a potential natural sleep aid for improving sleep in adults with sleep disturbance.
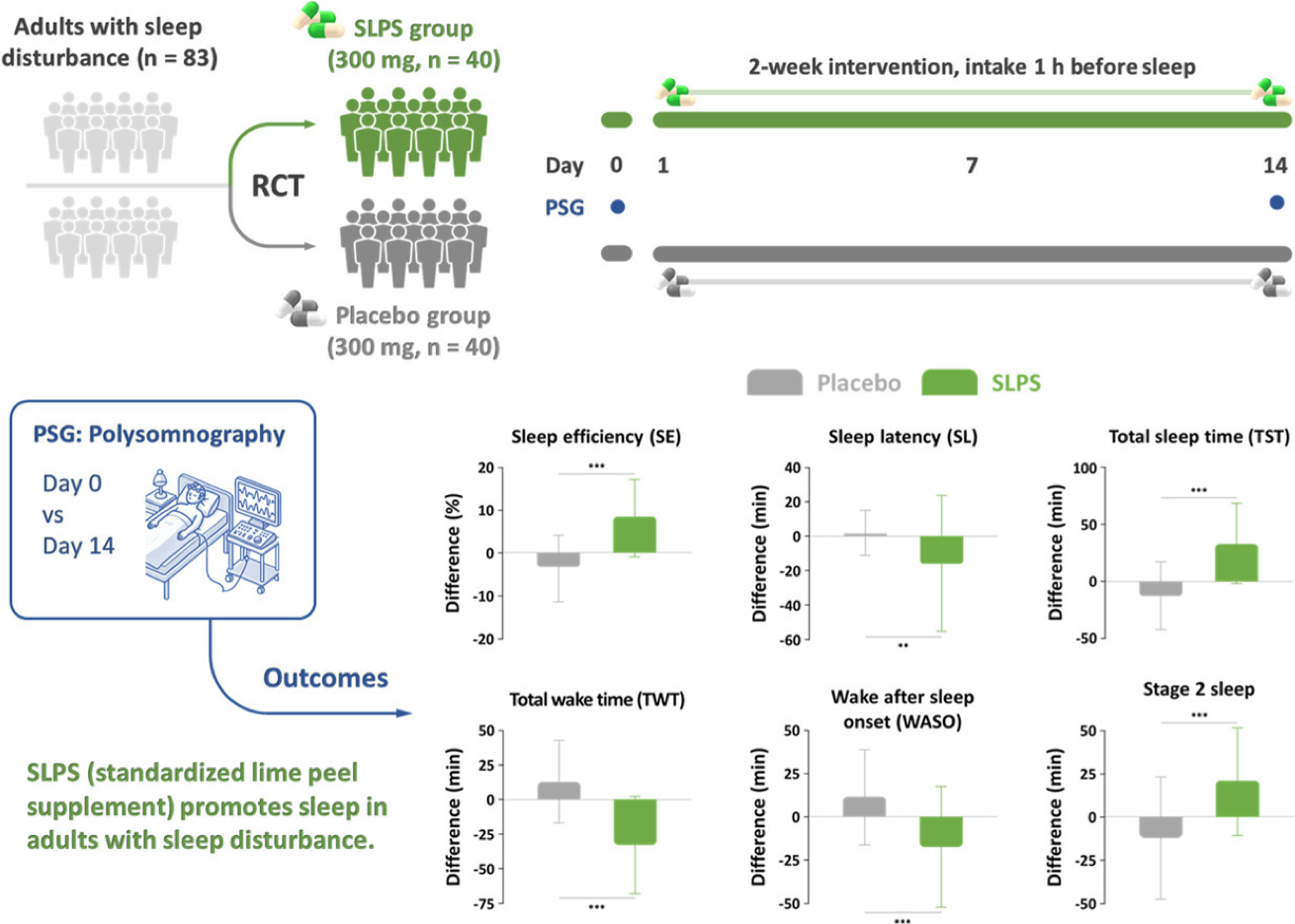
Source: Kim et al., Efficacy and safety of standardized lime peel supplement in adults with sleep disturbance: A randomized, double-blind, placebo-controlled, polysomnographic study. Phytomedicine 139, 156510, 2025.
- Test substance: SLPS (standardized lime peel supplement, BENESOMNOTM)
- Test animal: ICR mice
- Results: Administration of SLPS (100, 200, and 400 mg/kg) resulted in a dose-dependent decrease in sleep latency and increase in sleep duration. The effect of SLPS (400 mg/kg) was comparable to that of zolpidem (10 mg/kg).
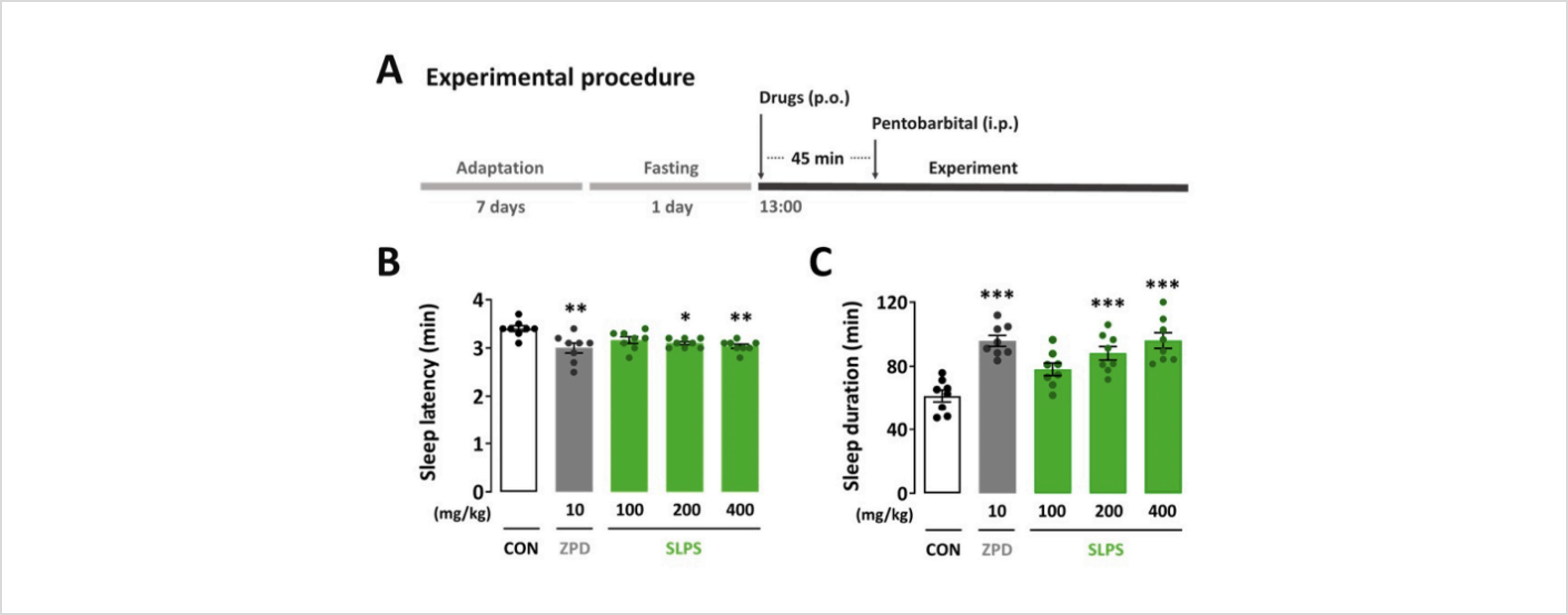
출처: Kim, S., Kim, D., Lee, J., Han, J. K., Um, M. Y., Jung, J. H., ... & Cho, S. (2024). Novel neuropharmacological activity of citrus lime (Citrus aurantifolia): A standardized lime peel supplement enhances non-rapid eye movement sleep by activating the GABA type A receptor. Biomedicine & Pharmacotherapy, 179, 117410.
- Test substance: SLPS (standardized lime peel supplement, BENESOMNOTM)
- Test animal: C57BL/6N mice
- Results: Administration of SLPS (100, 200, and 400 mg/kg) resulted in a dose-dependent decrease in sleep latency and increase in NREMS. Furthermore, no significant difference was observed between SLPS at 400 mg/kg and zolpidem at 10 mg/kg.
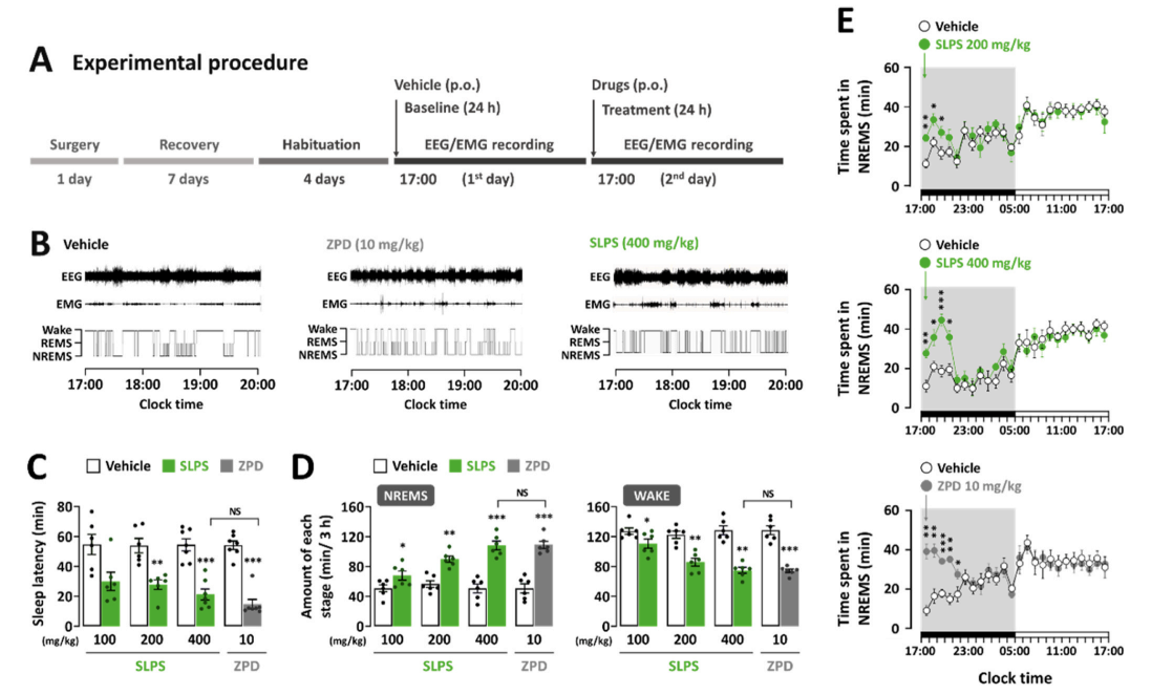
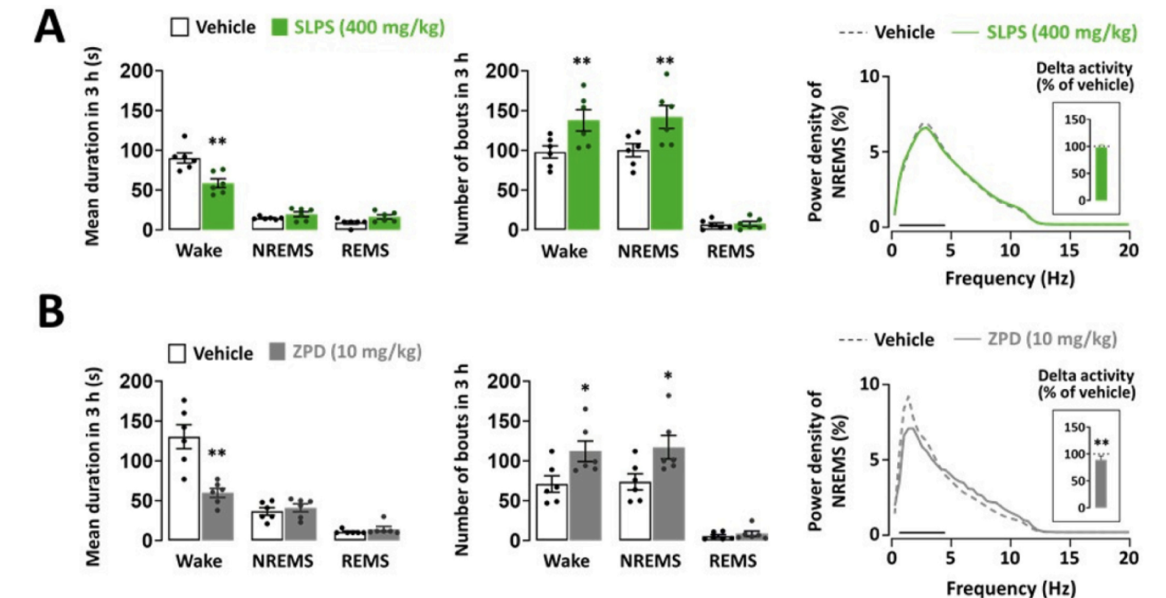
출처: Kim, S., Kim, D., Lee, J., Han, J. K., Um, M. Y., Jung, J. H., ... & Cho, S. (2024). Novel neuropharmacological activity of citrus lime (Citrus aurantifolia): A standardized lime peel supplement enhances non-rapid eye movement sleep by activating the GABA type A receptor. Biomedicine & Pharmacotherapy, 179, 117410.
- SLPS (standardized lime peel supplement) exerts sleep-promoting effects by modulating the GABAA receptors. When active compounds in SLPS binds to the GABA-binding site of the GABAA receptors, it enhances receptor activation, leading to a large influx of Cl- ions into the neuronal cells. This results in hyperpolarization, which inhibits neurotransmission and induces sedative and sleep-promoting effects.
- SLPS inhibited the binding of [3H]-muscimol, a well-known GABAA receptor agonist, in a dose-dependent manner. The IC50 value of SLPS was determined to be 0.026 mg/mL.
- SLPS induced GABAA receptor activity. The heightened responses to the SLPS were almost blocked by the GABAA receptor antagonist bicuculline, confirming the specificity of the observed effects on GABAA receptor activity.
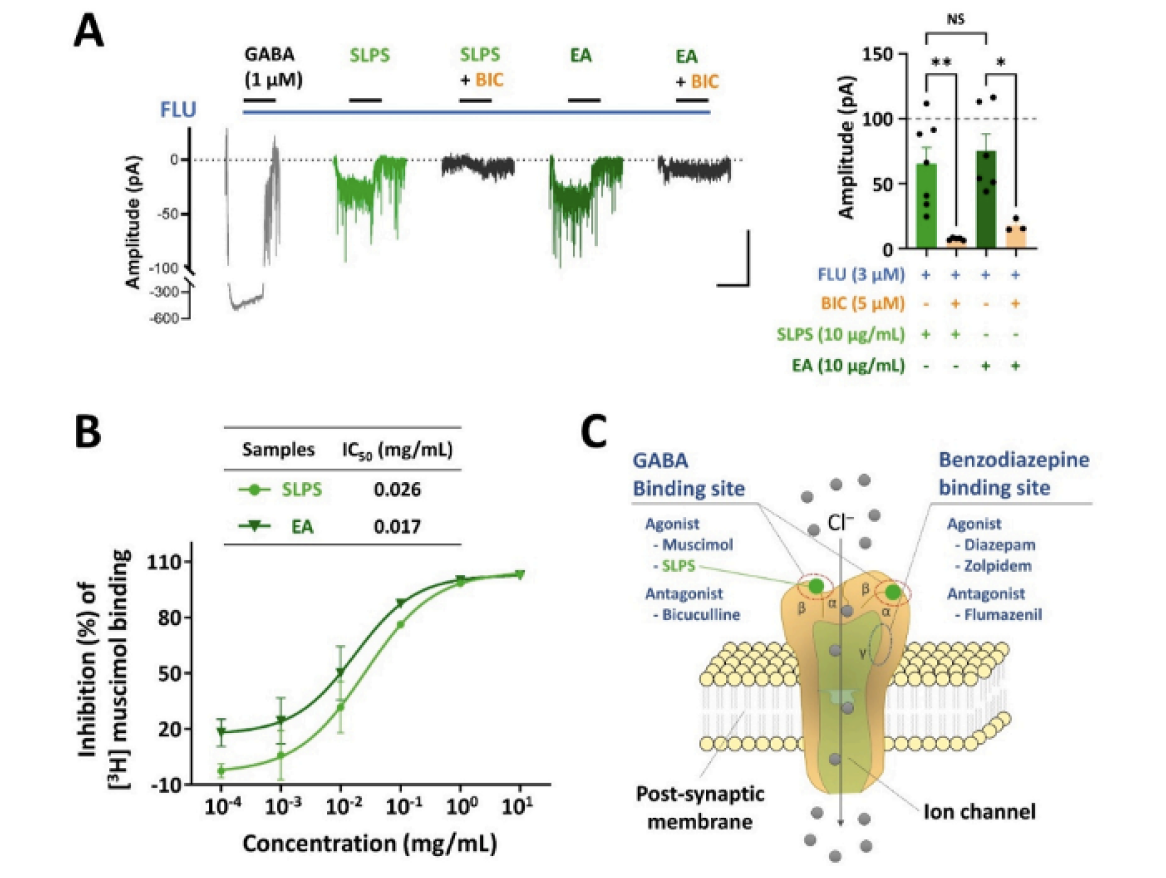
Source: Kim, S., Kim, D., Lee, J., Han, J. K., Um, M. Y., Jung, J. H., ... & Cho, S. (2024). Novel neuropharmacological activity of citrus lime (Citrus aurantifolia): A standardized lime peel supplement enhances non-rapid eye movement sleep by activating the GABA type A receptor. Biomedicine & Pharmacotherapy, 179, 117410.
- In the pentobarbital-induced sleep test and sleep structure analysis, the hypnotic effect of SLPS was fully blocked by bicuculline, similar to muscimol, a GABAA receptor agonist.
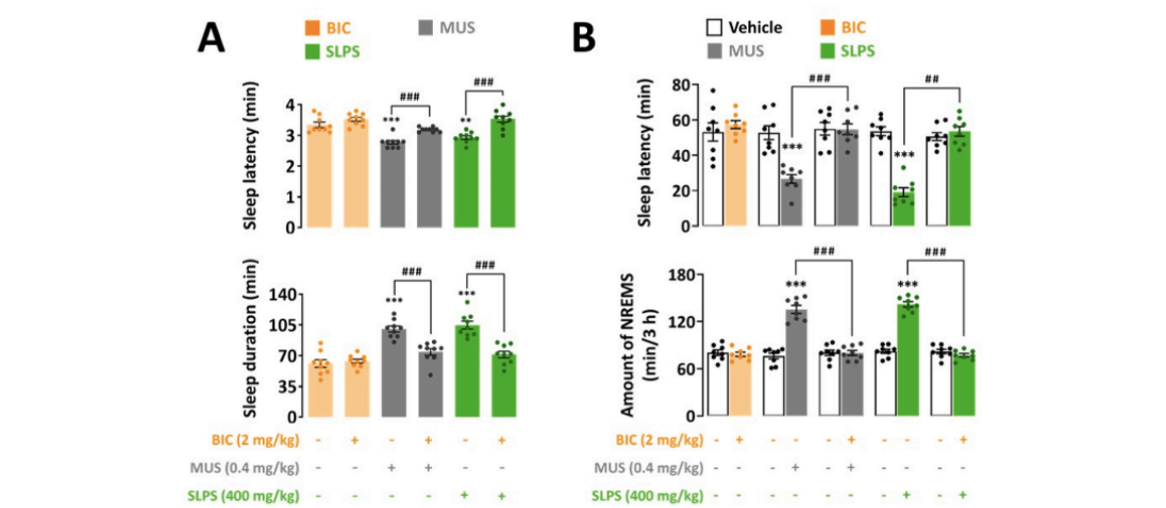
출처: Kim, S., Kim, D., Lee, J., Han, J. K., Um, M. Y., Jung, J. H., ... & Cho, S. (2024). Novel neuropharmacological activity of citrus lime (Citrus aurantifolia): A standardized lime peel supplement enhances non-rapid eye movement sleep by activating the GABA type A receptor. Biomedicine & Pharmacotherapy, 179, 117410.
Both the toxicity studies in human and animals showed no adverse affects, confirming that long-term and medium-term consumption of the BENESOMNO™ at the daily intake dose (300 mg/day) is safe without any safety concerns.
| Toxicity studies in humans |
Toxicity studies in humans
|
| Toxicity studies in animals |
Toxicity studies in animals
|