Nutra-It possesses extensive experience and expertise in the R&D and regulatory approval of functional ingredients. Furthermore, we take a balanced approach in our development process, carefully considering both the scientific aspects essential for nutraceuticals and health functional foods, as well as the industrial requirements.
for Functional Ingredient Development
Objective efficacy evaluation
Outstanding bioactivity
Identification of active compounds
Elucidation of mechanisms of action
Human clinical trial design
Securing patents
Economic feasibility (supply stability, cost, etc.)
Differentiation (raw materials, storytelling, etc.)
Consumer demand
Global expansion (international joint research)
International

Keck School of Medicine University of Southern California

The University of Tokyo

Fudan University
Domestic

Institute for Basic Science

Korea Institute of Science and Technology

Korea Functional Food Institute
| No. | Project Name | Supporting Agency | Period | Budget (Million KRW) |
|---|---|---|---|---|
| 3 |
TIPS Overseas Marketing
Support Project |
Ministry of SMEs and Startups | 2024.11 ~2025.08 |
80 |
| 2 | TIPS Program | Ministry of SMEs and Startups | 2022.07 ~2024.12 |
500 |
| 1 | Early Startup Package | Ministry of SMEs and Startups | 2021.05 ~2022.02 |
80 |




| No | Certificate | Invention Title | Application/Registration | Registration (Application) Number | Date |
|---|---|---|---|---|---|
| 1 |

|
Composition for the prevention, improvement or treatment of
sleep disorders or insomnia containing extract of roasted citrus by-product |
Registration | 제10-2569228호 | 2023. 08. 17 |
| No | Filing Country | Certificate | Invention Title | Application/Registration | Registration (Application) Number | Date |
|---|---|---|---|---|---|---|
| 1 | Usa |

|
COMPOSITION FOR PREVENTING, AMELIORATING OR TREATING SLEEP DISORDER OR INSOMNIA, CONTAINING EXTRACT OF ROASTED CITRUS BYPRODUCT AS ACTIVE INGREDIENT |
Application | 18033699 | 2023. 04. 25 |
| 2 | China |

|
COMPOSITION FOR PREVENTING, AMELIORATING OR TREATING SLEEP DISORDER OR INSOMNIA, CONTAINING EXTRACT OF ROASTED CITRUS BYPRODUCT AS ACTIVE INGREDIENT |
Application | 202280020678.9 | 2023. 10. 09 |
| 3 | Europe |

|
COMPOSITION FOR PREVENTING, AMELIORATING OR TREATING SLEEP DISORDER OR INSOMNIA, CONTAINING EXTRACT OF ROASTED CITRUS BYPRODUCT AS ACTIVE INGREDIENT |
Application | EP22911933.4 | 2023. 05. 01 |
Nutra-It develops functional ingredients for health supplements and provides research and development services, including sleep functionality efficacy evaluations and individual approval consulting. Contact us anytime via phone or email for inquiries and consultations. Details on sleep functionality efficacy evaluations are as follows
Biomarker assessment for verifying sleep health functionality (in accordance with the Ministry of Food and Drug Safety’s Guidelines for functionality evaluation of functional ingredients)
| Category | Biomarker | Measurable Research Type | |||
|---|---|---|---|---|---|
| In Vitro | In vivo | In Human | |||
| Sleep-related neurotransmitter analysis |
GABA, serotonin, orexin, etc. | O | O | O | |
| Biochemical markers | Melatonin in blood/saliva, 6-SMT in urine | O | O | ||
| Sleep evaluation | Neurophysiological monitoring |
PSG, EEG/EMG | O | O | |
| Sleep questionnaire | O | ||||
| Sleep actigraphy | O | O | |||
| Sleeping position monitoring |
Accelerometer | O | O | ||
| Sleep questionnaire |
PSQI (Pittsburgh Sleep Quality Index) ISI (Insomnia Severity Index) Sleep diary |
O | |||
※출처: 건강기능식품 기능성 평가 가이드(수면건강 관련), 식품의약품안전처, 2022.
- Sleep is a behavioral phenomenon that cannot be directly assessed at the cellular level, making animal models essential
- The pentobarbital-induced sleep test, using pentobarbital anesthesia, is a widely used model for evaluating sleep functionality
- This animal model utilizes the characteristic that mice administered a sleep aid (or functional sleep ingredient) before anesthesia fall asleep faster (significantly reduced sleep latency) and sleep longer (significantly increased sleep duration) compared to the control group
- The detailed experimental procedure of the pentobarbital-induced sleep test is illustrated in the following schematic diagram
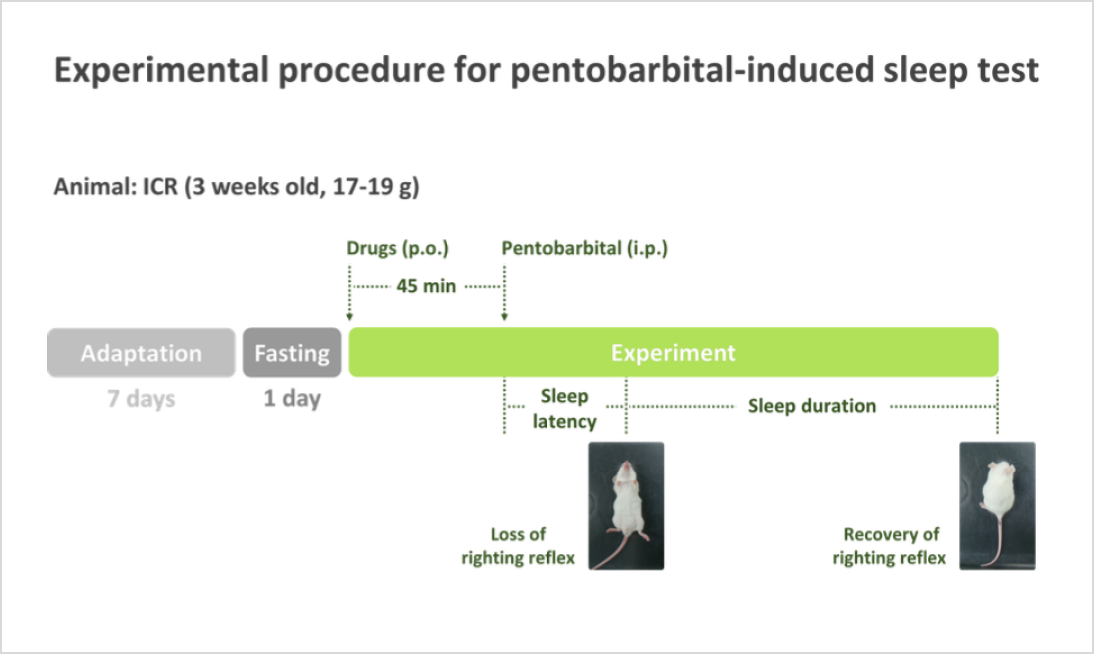
- (Sleep latency) The time taken to fall asleep, one of the most well-known biomarkers for sleep functionality evaluation.
- (Sleep duration) It refers to the period from the loss of the righting reflex until its recovery, and is widely used together with sleep latency as a key biomarker for sleep functionality evaluation
- (Rationale) Difficulty falling asleep or maintaining sleep indicates potential sleep issues. Therefore, a decrease in sleep latency and an increase in sleep duration are key biomarkers for evaluating sleep improvement.
- (Measurement method) Sleep latency is measured as the time from pentobarbital injection to the loss of the righting reflex, while sleep duration is the time from the loss of the righting reflex until its recovery.
- Analysis of sleep architecture animal model utilizes mice or rats, as these rodents share similarities with humans in terms of sleep homeostasis regulation, circadian rhythm, and sensitivity to internal and external stimuli
- Unlike other health-functional animal models, which induce symptoms, this model directly evaluates sleep functionality, as rodents naturally experience fragmented sleep, making them an inherent insomnia model
- Therefore, the functionality evaluations of sleep aids and functional sleep ingredients published in research studies have been conducted without inducing an insomnia model
- In the analysis of sleep architecture animal model, evaluations are conducted in a specialized chamber with lightweight mice, recording electroencephalogram (EEG) and electromyogram (EMG) to analyze sleep structure, making it the most systematic and objective method for assessing sleep functionality
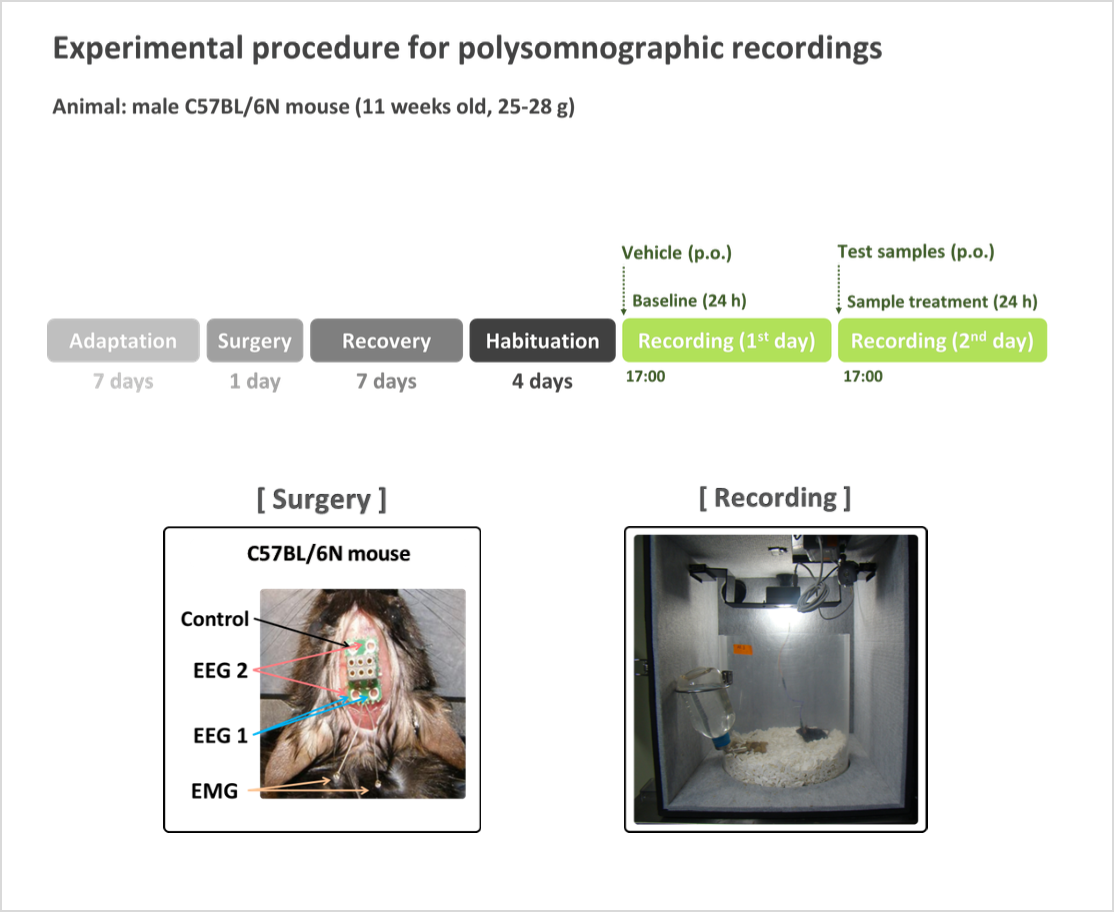
- (Sleep latency) The time taken to fall asleep, one of the most well-known biomarkers for sleep functionality evaluation.
- (Sleep duration of NREMS) Sleep is classified into rapid-eye movement sleep (REMS) and non-rapid eye movement sleep (NREMS). REMS is associated with dreaming and wake-like brain activity, while NREMS is generally considered an indicator of deep sleep.
- (Rationale) Difficulty falling asleep or maintaining sleep indicates potential sleep issues. Therefore, a decrease in sleep latency and an increase in sleep duration are key biomarkers for evaluating sleep improvement.
- (Measurement method) After administering the functional sleep ingredients, 24-hour EEG and EMG recordings are analyzed using sleep architecture evaluation programs (e.g., SleepSign) to classify NREMS, REMS, and wakefulness, providing a comprehensive assessment of sleep architecture.


 />
/>